What Is The Electron Configuration For Aluminum
What Is The Electron Configuration For Aluminum. Aluminum is one of the elements that make up the periodic table, which is distinguished by its symbol al, and its atomic number. The aluminium atom and si +1, p +2, s +3, cl +4 have the same electronic configuration.
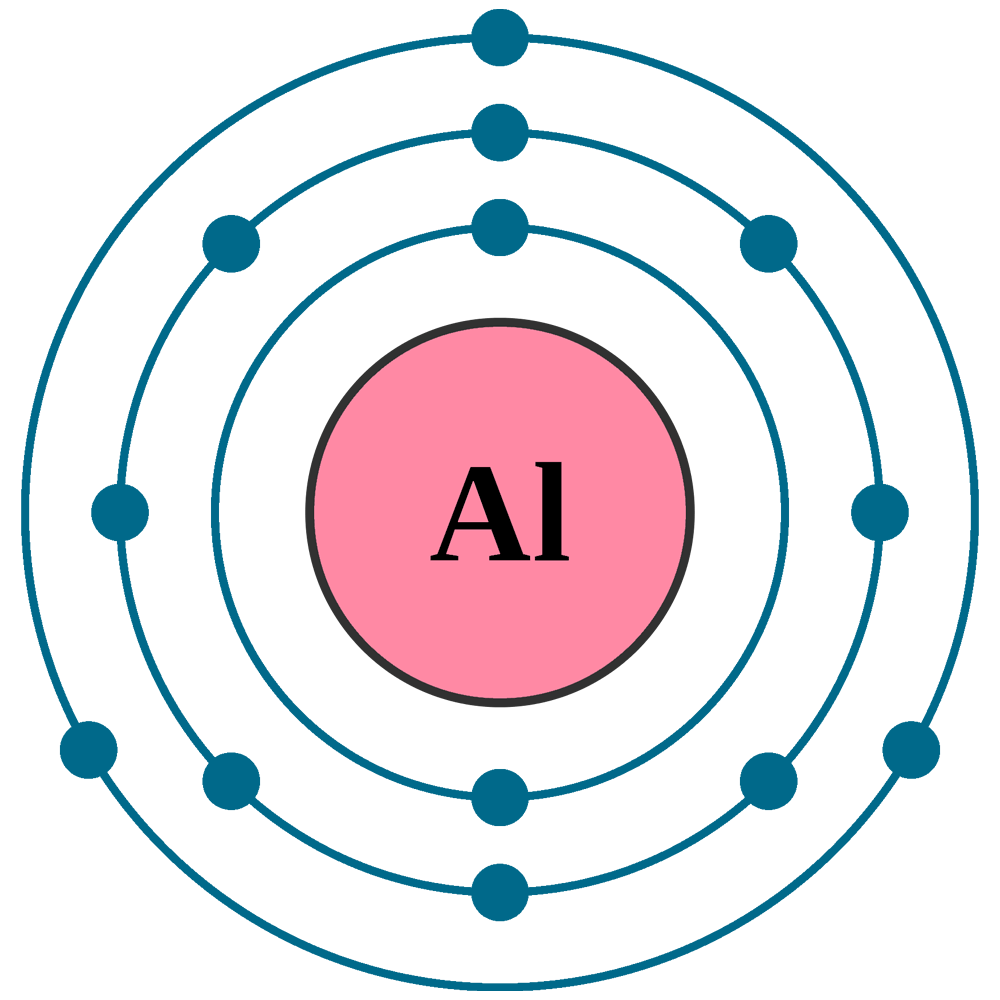
The electron configuration for aluminum is 1s 2 2s 2 2p 6 3s 2 3p 1. The electron configuration of aluminum is 1s2 2s2 2p6 3s2 3p1. Aluminum is one of the elements that make up the periodic table, which is distinguished by its symbol al, and its atomic number.
The Arrangement Of Electrons Into The Orbitals Of An Atom Using Some Fundamental Principle Is Called Its Electronic Configuration.
We’ll also look at why aluminum forms a 3+ ion and how the electron confi. Aluminum ion, al3+, is in noble gas configuration (between '[ ]' ) is [1s2, 2s2 2p6] 3s0 3p0 aluminum's. The electron configuration of an aluminum(al) atom can be done in two ways.
(The Condensed Electron Configuration Of Aluminum Is [Ne] 3S2 3P1.)
Aluminium has good corrosion resistivity. Electron configuration through orbit (bohr principle) electron configuration through orbital (aufbau principle) electron configuration through orbitals follows different principles. Nevertheless, check the complete configuration and other interesting facts about aluminum.
Therefore The Phosphorus Electron Configuration Will Be 1S22S22P63S23P3.
The electron configuration of aluminum is 1s22s22p63s23p1. The chemical symbol for aluminium is al. Study with quizlet and memorize flashcards containing terms like what is the electron configuration for aluminum?, which of the following electron configurations is impossible?.
It Is Capable Of Superconductivity.
In order to write the aluminium electron configuration we first need to know the number of electrons for the al atom (there are 13 electrons). We know, the electron configuration of the aluminum atom is 1s 2 2s 2 2p 6 3s 2 3p 1, and valence electrons are those electrons found in the outer shell of an atom. Electronic configuration of aluminium in short form:
The Electron Configuration Of Aluminum Is 1S2 2S2 2P6 3S2 3P1.
The aluminium atom and si +1, p +2, s +3, cl +4 have the same electronic configuration. [ne] 3s 2 3p 1; The ground state electron configuration is [ne]3s 2 3p 1.
Post a Comment for "What Is The Electron Configuration For Aluminum"